
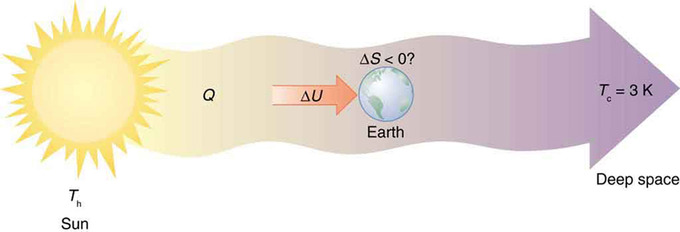
We might visualize that with the mailbox analogy we used for entropy earlier. That's because the system is just everything involved in the reaction, and the surroundings are everything that isn't involved in the reaction.Įnthalpy changes in the system lead to additional partitioning of energy. Together, the system and the surroundings are called "the universe". In an endothermic reaction, the external entropy (entropy of the surroundings) decreases.įree energy takes into account both the entropy of the system and the entropy changes that arise because of heat exchange with the surroundings.In an exothermic reaction, the external entropy (entropy of the surroundings) increases.So far, we have just considered internal entropy changes. Specifically, the internal enthalpy change that we discussed earlier has an effect on the entropy of the surroundings. Thus, an enthalpy change can also have an effect on entropy. The reason for this relationship is that if energy is added to or released from the system, it has to be partitioned into new states.
As it happens, enthalpy and entropy changes in a reaction are partly related to each other. Sometimes it is said that entropy governs the universe.
IN WHICH OF THESE SYSTEMS IS THE ENTROPY DECREASING FREE
Endergonic ones (in which free energy increases) are not. In free energy terms, we say that exergonic reactions are favored(in which free energy decreases). Exothermic reactions were favored (in which enthalpy decreases). Remember the terms "endothermic" and "exothermic" from our discussion of enthalpy. Both factors have already been taken into account.
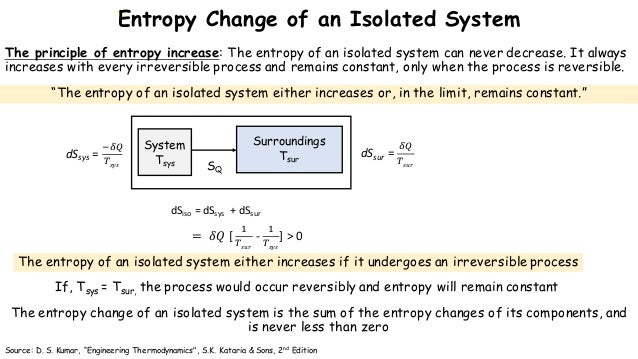
The temperature acts as a scaling factor in the expression, putting the entropy and enthalpy on equivalent footing so that their effects can be compared directly. \(T\) in this expression stands for the temperature (in Kelvin, rather than Celsius or Fahrenheit). Mathematically, the symbol for the internal enthalpy change is "ΔH" and the symbol for the internal entropy change is "ΔS." Free energy is symbolized by "ΔG," and the relationship is given by the following expression: The combined effects of enthalpy and entropy are often combined in what is called "free energy." Free energy is just a way to keep track of the sum of the two effects.
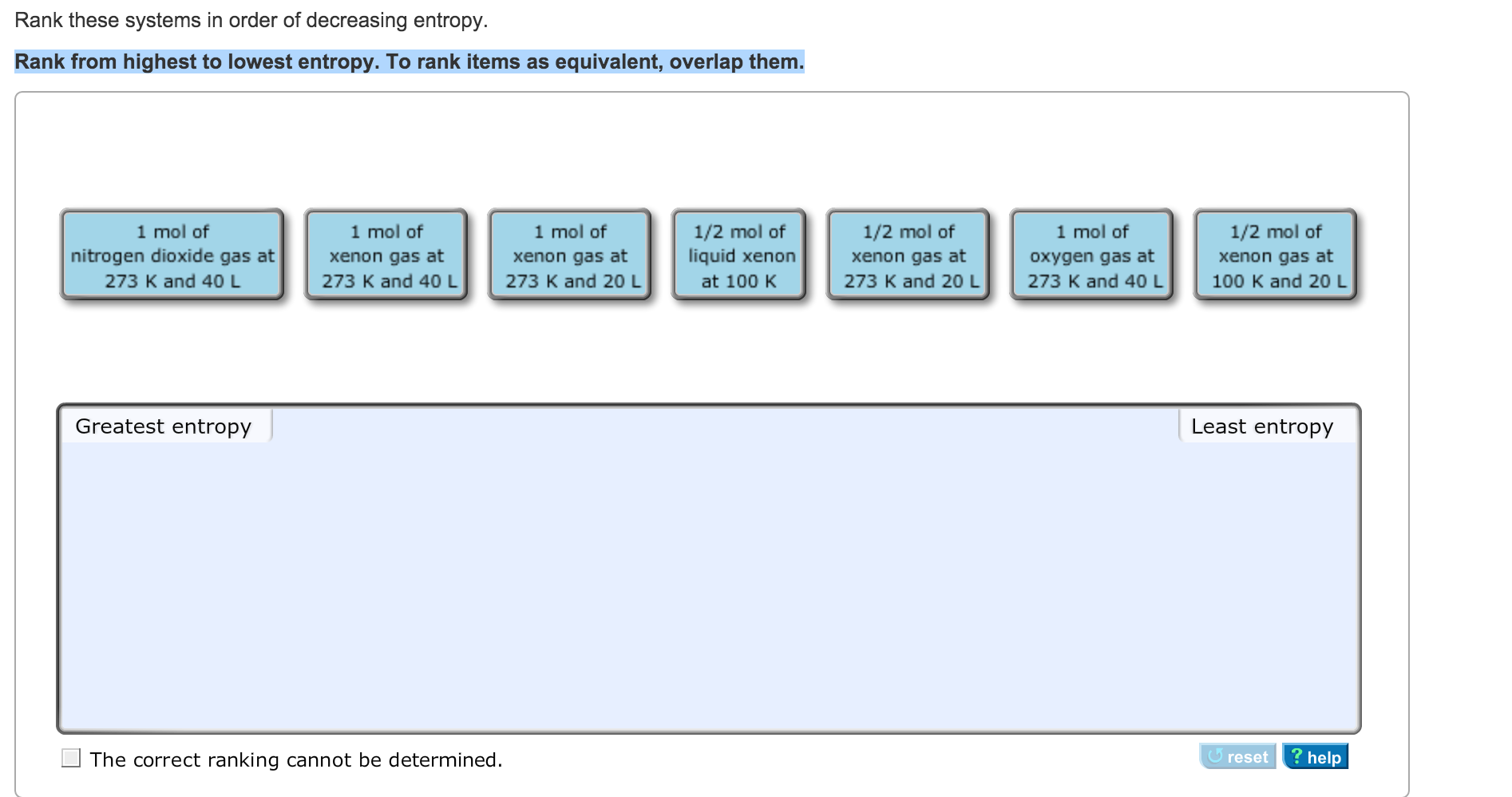
How much does the enthalpy decrease? How much does the entropy decrease? If the effect of the enthalpy decrease is greater than that of the entropy decrease, the reaction may still go forward. For example, what if enthalpy decreases, but so does entropy? Does the reaction happen, or doesn't it? In that case, we may need quantitation to make a decision. Having two factors may lead to complications. In this case, the red squares will remain just as they are. Both factors tilt the balance of the reaction to the left. If we think of the balance between these two factors, we come to another simple conclusion. In this case, the red squares will be converted into green circles.Īlternatively, maybe entropy decreases when the red squares turn into green circles, and enthalpy increases. Both factors tilt the balance of the reaction to the right. If we think of the balance between these two factors, we come to a simple conclusion. In one case, maybe entropy increases when the red squares turn into green circles, and the enthalpy decreases. Whether or not the reaction proceeds to the right depends on the balance between enthalpy and entropy. Red squares are being converted to green circles, provided the reaction proceeds from left to right as shown. Reactions can happen when entropy increases.Ĭonsider the cartoon reaction below. A reaction is favored if entropy increases: There is also a bias in nature toward increasing entropy in a system.Reactions can happen when enthalpy is transferred to the surroundings. A reaction is favored if enthalpy decreases: There is a bias in nature toward decreasing enthalpy in a system.Entropy has something to do with how that energy is stored. Enthalpy has something to do with the energetic content of a system or a molecule. \)Įntropy and enthalpy are two of the basic factors of thermodynamics.


 0 kommentar(er)
0 kommentar(er)
